Select the Statement That Best Describes a Buffer S
The pH of a buffer solution is determined by the ratio of the concentration of conjugate base to the concentration of. B A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic.
A weak base B and its conjugate acid HB.
. Concept 33 A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added. - A buffer stabilizes the pH of a solution by preventing acids or bases from dissociating.
A buffer is a solution made by combining either. C Buffer resists change in pH by accepting hydrogen ions when acids are added to the solution. O A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic.
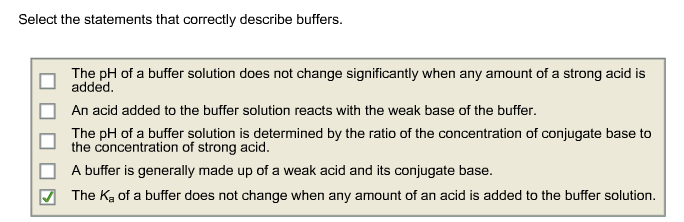
The pH of a buffer solution does not change significantly when any amount of a strong acid is added. - A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added. Select the statement that best describes a buffer.
The greatest amount of acid or base that a buffer can accept while maintaining pH is called the buffer capacity. O A buffer prevents the pH of a solution from changing when an acid or base is added. A A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added.
AA buffer prevents the pH of a solution from changing when an acid or base is added. An acid added to the buffer solution reacts with the weak base of the buffer. An acid added to the buffer solution reacts with the weak base of the buffer.
A buffer stabilizes the pH of a solution by preventing acids or bases from dissociating. A buffer prevents the ph of a solution from changing when an acid or base is added. Select the statements that correctly describe buffers.
2 The Ka of a buffer does not change when any amount of an acid is added to the buffer solution. Select the statements that correctly describe buffers 厂 The pH of a buffer solutions determined by the ratio of the concentration of con gate base to the concentration of strong acid. Select the statements that correctly describe buffers.
THIS IS THE BEST ANSWER. A buffer is generally made up of a weak acid and its conjugate base. AA buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic.
A weak acid HA and its conjugate base A. Buffer resists change in pH by accepting ttydrogen ions when acids are added to the solution and donating. A buffer accepts hydrogen ions when they are in excess and donates hydrogen ions when they have.
The K_a of a buffer decreases. O Abuffer resists change in pH by accepting ttydrogen ions when acids are added to the solution and donating hydrogen ions. Select the statement that best describes a buffer.
A Select the statement that best describes a buffer. 1 The pH of a buffer solution does not change significantly when any amount of a strong acid is added. A buffer prevents the pH of a solution from changing when an acid or base is added.
A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. The statement that best describes a buffer is. Select the statement that best describes a buffer.
DA buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added. Part A Select the statement that best describes a buffer. B A buffer prevents the pH of a solution from changing when an acid or base is added.
The resistance of the solution is called Buffer Capacity and the solutions are called Buffer solutions. Select the statement that best describes a buffer. Select the statements that correctly describe buffers.
Added An acid added to the buffer solution reacts with the weak base of the buffer. Select the best choice from these four acids to start making a buffer nearest the desired pH. Once the buffer capacity is exceeded in cells key functions of the body can be disrupted.
A buffer is generally made up of a. - A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. The K_a of a buffer does not change when any amount of an acid is added to the buffer solution.
O A buffer prevents the pH of a solution from changing when an acid or base is added. A buffer solution together with its salt usually contains a. The pH of a buffer solution is determined by the ratio of the concentration of acid to the concentration of base.
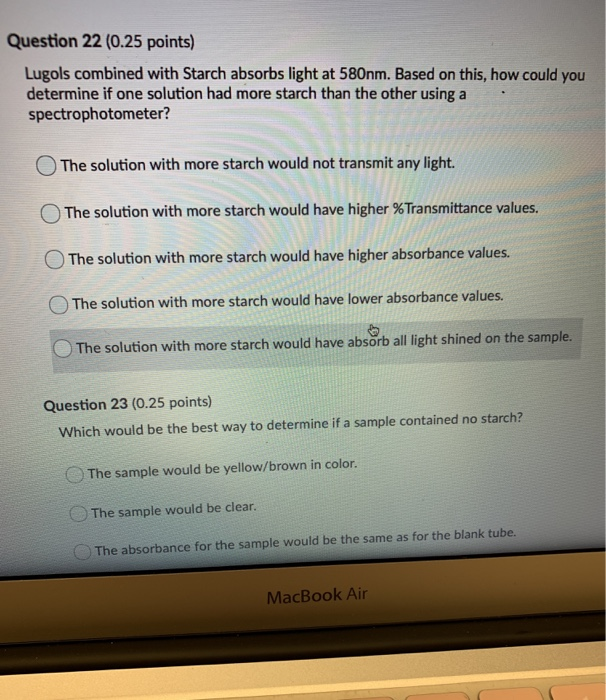
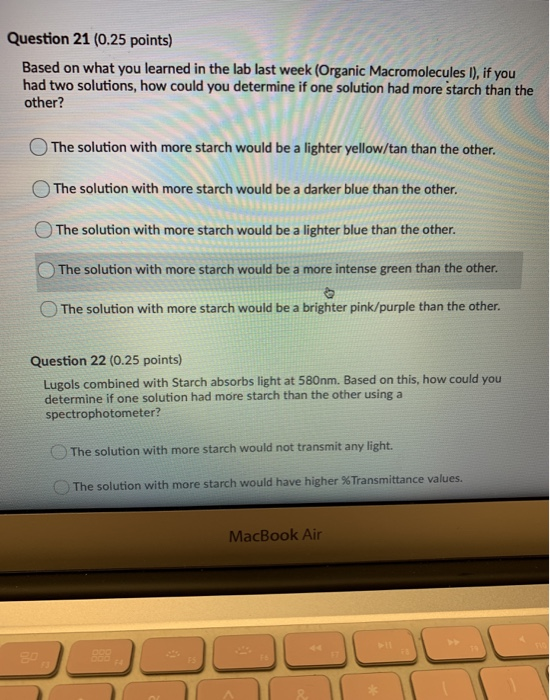
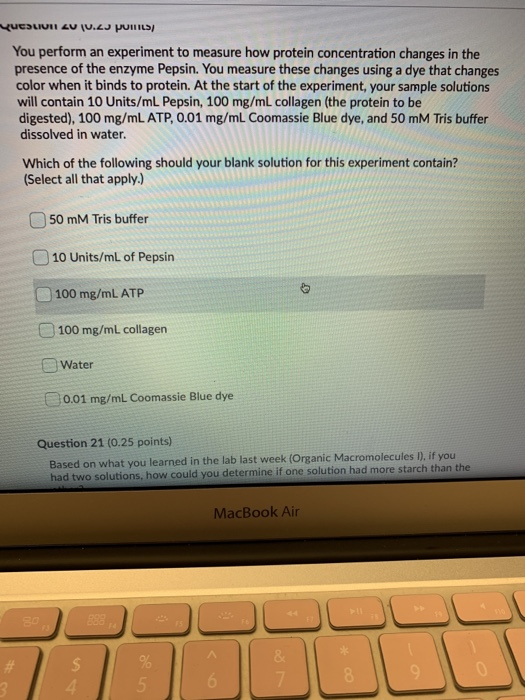
Biology questions and answers. The pH of a buffer solution does not change significantly when a small amount of acid is added. A buffer resists change in pH by accepting hydrogen ions when acids are added to the solution and donating hydrogen ions when bases are added.
O A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic. The solution on the right is basic relative to the solution on the left. A buffer causes acidic solutions to become alkaline and alkaline solutions to become acidic.
A buffer is made up of a strong acid and a strong base. BA buffer stabilizes the pH of a solution by preventing acids or bases from dissociating. A buffer stabilizes the ph of a solution by preventing acids or bases from dissociating.
Up to 256 cash back 11 Dec 2019. Select the statement that best describes a buffer. A buffer prevents the pH of a solution from changing when an acid or base is added.
A certain solution has the ability to resist a significant change in the concentration of hydrogen ions ie pH when a small amount of acid or base is added to these solutions. Buffered solutions are always neutral with a pH of 7. Select the statements that correctly describe buffers.
Hydrofluoric acid HF K 68x10-4 Formic acid HCHO Kg 17x10-4 Acetic acid HCHCO K 17x10-5 Propionic acid HC2H50 Ks 13x10-5 Assume the best acid is combined with its appropriate conjugate base. A Select the statement that best describes a buffer. The higher the concentration of weak acid and conjugate base in solution the higher the buffer capacity.
View available hint s select the statement that best describes a buffer. A buffer stabilizes the pH of a solution by preventing acids or bases from dissociating. Buffered solutions are always neutral with a ph of 7.
Solved Question 19 0 25 Points Which Statement Best Chegg Com

Ap Biology Semester 1 Final Review Flashcards Quizlet
Solved Question 19 0 25 Points Which Statement Best Chegg Com

Solved Select The Statements That Correctly Describe Chegg Com
Solved Question 19 0 25 Points Which Statement Best Chegg Com
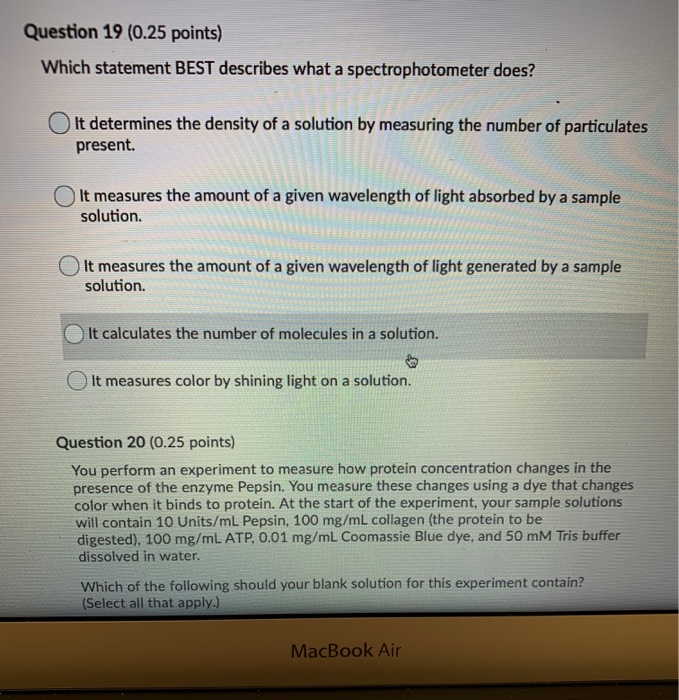
Solved Question 19 0 25 Points Which Statement Best Chegg Com

Ch 14 Test Bank Chapter 14 Shock And Multiple Organ Dysfunction Syndrome A Nurse In The Icu Is Studocu

Pdf Financial Well Being A Survey Of Adults In Australia

Solved Short Answer Write The Word Or Phrase That Best Chegg Com

Ap Bio Exam Flashcards Quizlet

Quiz Worksheet Steps In The Polymerase Chain Reaction Study Com

Which Statement Best Describes A Difference Between Electromagnetic Waves And Mechanical Waves A Brainly Com

Ap Bio Exam Flashcards Quizlet
Sql Examples For Beginners Sql Select Statement Usage
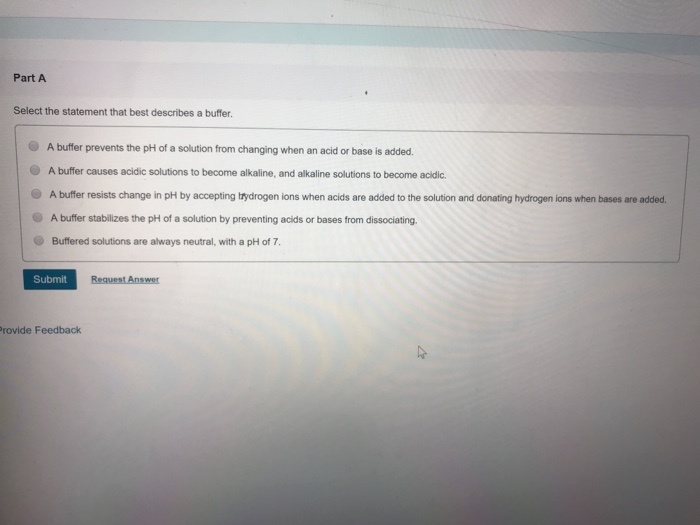
Solved Part A Select The Statement That Best Describes A Chegg Com

Biol 1406 Learning Curve Ch 8 Flashcards Practice Test Quizlet
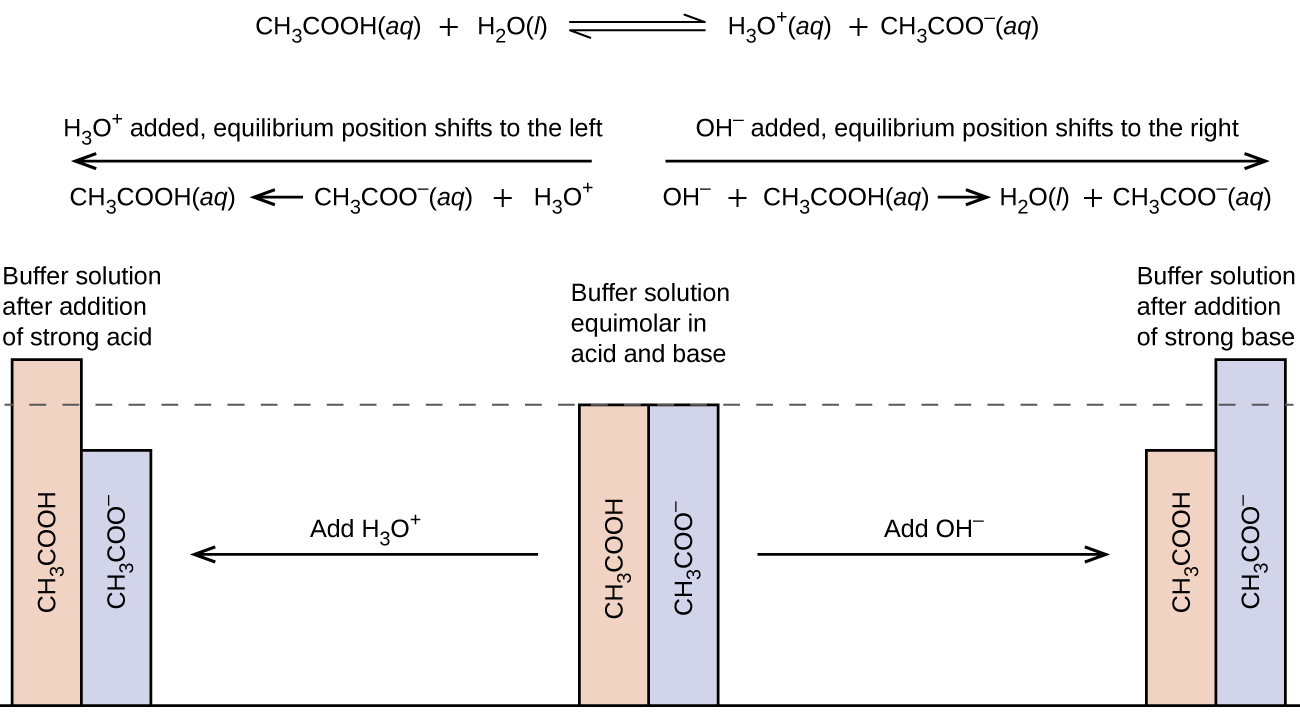
Comments
Post a Comment